Lithium-Ion Batteries
Research - Jul 16, 2024
Lithium-ion batteries (LIBs) are becoming a necessity in our lives compared to what they were only a decade ago. We use them in our phones, smart watches, laptops, power tools, vehicles…etc.
However, the convenience of having such a cool technology is confronted by the downside of having to recharge it after using it for some time. What is even worse is that the battery gets drained faster if the embedding device operates at a high load. For example, we do not need to recharge our phone as often if we simply use it for texting compared to when using it for web browsing. This is because web browsing is more resource demanding than texting.
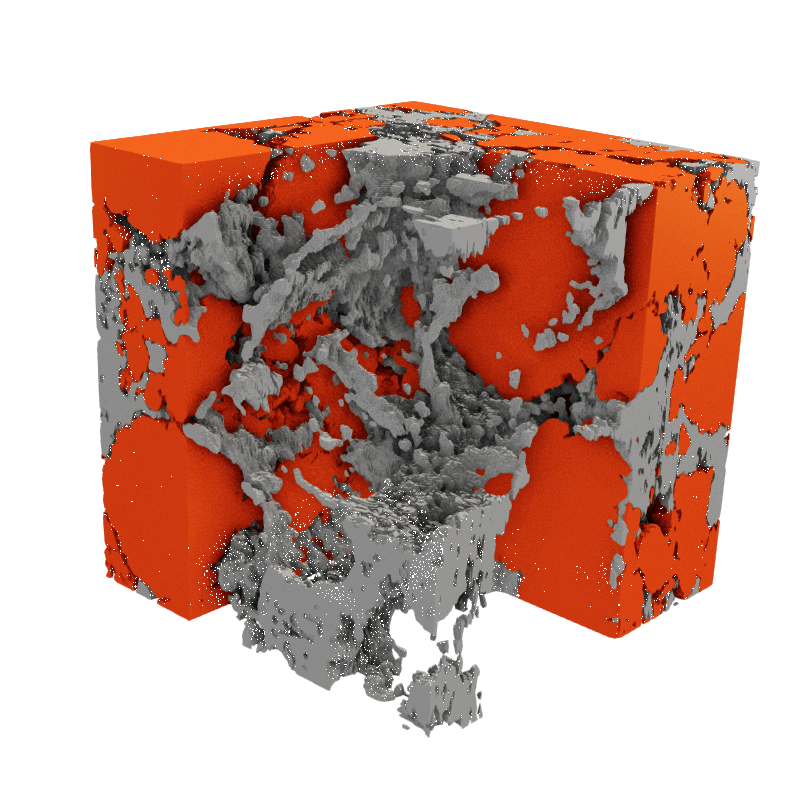
What controls this behavior is the battery’s microstructure. This microstructure has the size of a human hair, and it represents a very detailed view of the battery when seen under a high-resolution microscope. This microstructure is composed of three main components:
- the active material (red) which stores lithium
- the conductive binder domain (CBD) which is an aggregation of conductive additives and binder material (gray) that allows the movement of electrons and binds the electrode together
- the electrolyte-filled pores that facilitate the transport of lithium ions during the battery’s operation.
The arrangement of these components in the microstructure dictates the amount of energy that can be extracted from the battery. It also determines how long a battery can operate before having to recharge it and how long it can last before being unusable. In this field of research, we try to understand how the quantities and arrangement of the microstructure components impact the battery’s performance with the aim of finding an optimal microstructure that results in the best performance.